Covalent Compounds Examples List
These covalent bonds are very strong. The polarity of the covalent bonds in water explains its solvent capabilities high boiling point high specific heat capacity surface.
Dative Bonding Co Ordinate Covalent Compound Chemistry Study
For example water is a covalent polar compound.
Covalent compounds examples list. Typically a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table except that hydrogen is almost never written first h 2 o is the prominent exception. Then the other nonmetal symbols are li. The elements form a compound by sharing electrons resulting in an electrically neutral molecule.
The elements in n 2 o 4 are both nonmetals rather than a metal and a nonmetal. A covalent compound is a compound in which the atoms that are bonded share electrons rather than transfer electrons from one to the other. The polarity of a compound explains a number of its physical structure such as its 3 dimensional geometry intermolecular interactions and phase behavior.
You would find ionic rather than covalent bonds in a salt such as sodium chloride. In this atomic molecule two hydrogen atoms share their single electrons with the oxygen atom which shares its own two electrons in return. Therefore the atoms form covalent bonds.
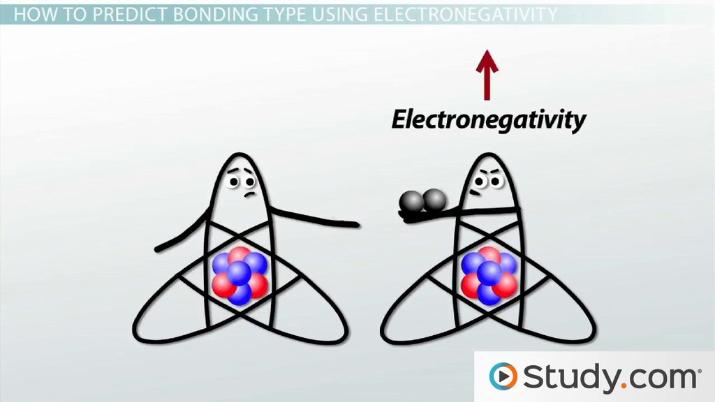
These were some illustrative examples which should have given you an idea about the nature of this type of chemical bond. As discussed before the sharing of electrons between the atoms which constitute the molecule is influenced by their individual electronegativity. Water consists of a covalent bond containing hydrogen and oxygen bonding together to make h 2 o.
The example of this kind of covalent compounds includes diamond and graphite of carbon atom network. They also tend to be very hard with high melting points which are different from most of the covalent compounds. You would find ionic rather than covalent bonds in a salt such as sodium chloride.
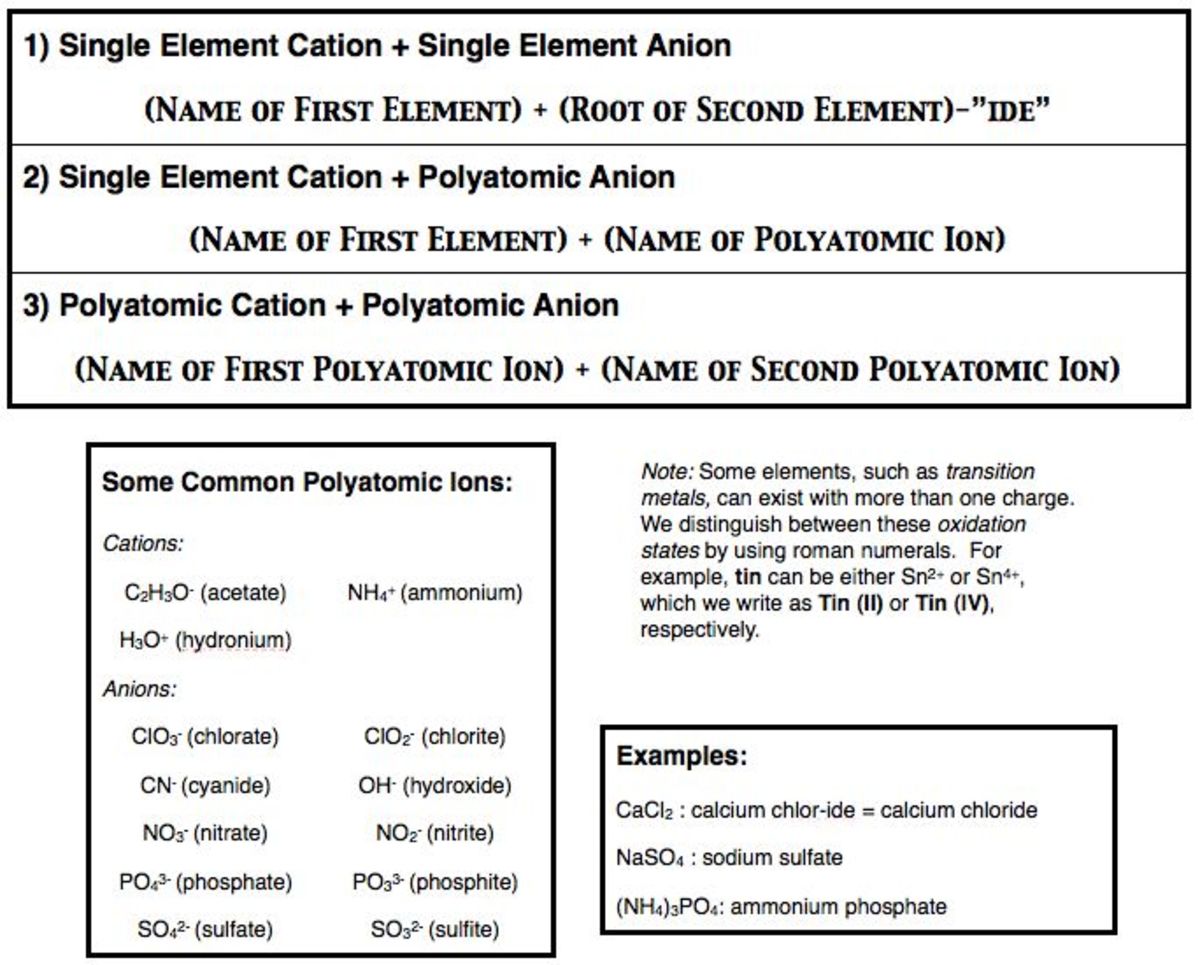
However polyatomic ions are held together by covalent bonds so this compound contains both ionic and covalent bonds. An example is water. The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate discrete molecules.
They also include silica of silicon and oxygen atoms network. So for example you would not expect to find covalent bonds in a metal or alloy such as silver steel or brass. For example if you want to mix an ionic compound or polar compound in an organic solvent you may be able to dissolve it in ethanol polar but not by a lot.
Then you can dissolve the ethanol solution into an organic solvent such as xylene. While ionic compounds are usually formed when metals bond. Covalent or molecular compounds generally result from two nonmetals reacting with each other.
Polar And Nonpolar Covalent Bonds Definitions And Examples

Chapter 8 Covalent Bonding 8 1 Molecular Compounds Ppt Video
Chem4kids Com Atoms Compounds

Covalent Compound Scavenger Hunt Key

Chemical Names And Formulas

Covalent Bond Examples Science Struck
Steps To Naming Ionic And Covalent Compounds Owlcation
Polar Covalent Compounds
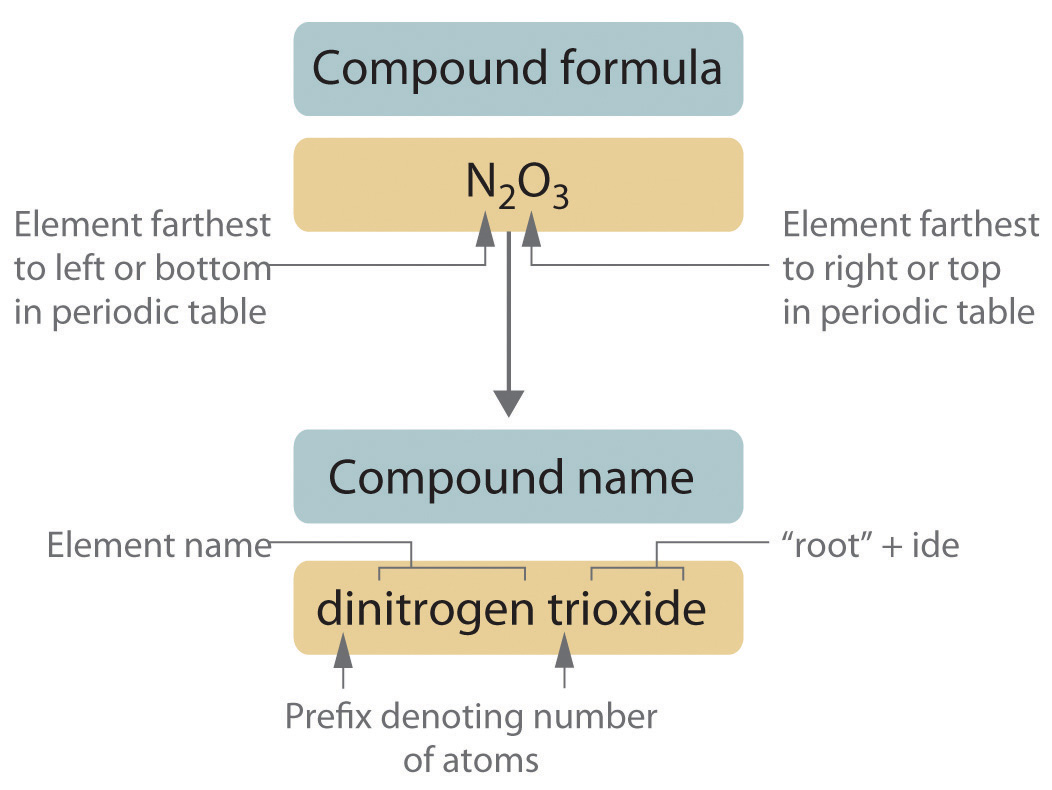
3 6 Naming Covalent Compounds Chemistry Libretexts
0 Response to "Covalent Compounds Examples List"
Post a Comment