Binary Ionic Compounds Definition
This is the definition of ionic compound along with examples of representative substances. Ionic compounds typically have high melting and boiling points and are hard and brittle.

What Is An Ionic Compound Formula And Defination Share Education
They do however conduct electricity when molten or when dissolved in solution when the ions are released from the crystal lattice and become mobile.

Binary ionic compounds definition. For a binary ionic compound a metal will always be the first element in the formula while a nonmetal will always be the second. We need you to answer this question. Ionic compounds dissolve best in polar.
Examples of binary ionic compounds include calcium chloride cacl 2 sodium fluoride naf and magnesium oxide mgo whilst examples of a binary covalent compounds include water h 2 o and sulfur hexafluoride sf 6. This is the definition of binary compound. Search the site go.
The compound is neutral overall but consists of positively charged ions called cations and negatively charged ions called anions. If you know the answer to this question please register to join our limited beta program and start the. This lesson will teach you the definition of a binary compound and go over several examples of binary compounds and non binary compounds so you can become a pro at spotting both.
Helmenstine holds a phd. A binary compound is a chemical compound that contains exactly two different elements. Binary covalent compoundscovalent compounds that contain only two elementsare named using a procedure similar to that used for simple ionic compounds but prefixes are added as needed to indicate the number of atoms of each kind.
Ionic compounds can also be produced from their constituent ions by evaporation of their solvent precipitation freezing a solid state reaction or the electron transfer reaction of reactive metals with reactive non metals such as halogen gases. This is the definition of ionic compound along with examples of representative substances. Naming binary ionic compounds when examining the formula of a compound in order to name it you must first decide what kind of compound it is.
In chemistry an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. Chemistry chemical laws basics molecules periodic table projects experiments scientific method biochemistry physical chemistry medical. In general ionic solids do not carry electric current because the ions are in fixed positions in the crystal lattice.
Solutions of ionic compounds. What is the definition of a binary ionic compound. Ionic compound what is an ionic compound.
In biomedical sciences and is a science writer educator and consultant.

What Is A Binary Compound Definition Examples Video
Binary Ionic Compound Calculator
Nomenclature Ionic And Covalent Lessons Tes Teach

Ferric Chloride Chemical Compound Britannica

Naming Compounds Boundless Chemistry
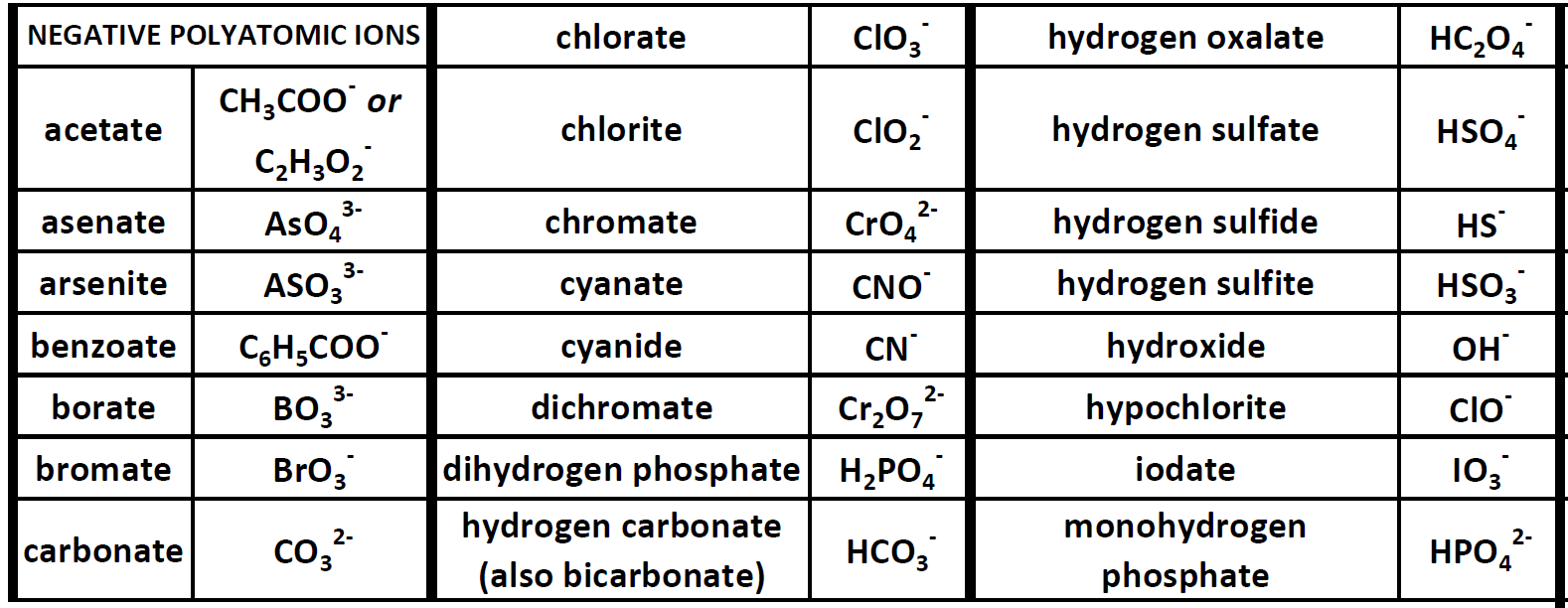
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry

Rules For Naming Ionic Compounds Video Lesson Transcript

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Ionic Compounds Formation Lattice Energy And Properties Video
0 Response to "Binary Ionic Compounds Definition"
Post a Comment