Ionic And Covalent Compounds Examples
These molecules have a slight negative charge on the oxygen atom and slight positive charges on the hydrogen. There are two types of bonds.
What Is The Difference Between Ionic Covalent And Metallic Bonds
Why are covalent compounds not soluble in water.
:max_bytes(150000):strip_icc()/covalent-bond-58de6d0b3df78c51627d1783.jpg)
Ionic and covalent compounds examples. You can recognize ionic compounds because they consist of a metal bonded to a nonmetal. Here are some differences. You would find ionic rather than covalent bonds in a salt such as sodium chloride.
If the compound contains a metal or the ammonium ion nh 4 it is ionic. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding. The list goes.
Many metal carbides are not ionic similarly nitrides. Both of these bonds are important in organic chemistry. Examples of covalent bonding include organometallic compounds which contain a metal carbon covalent bond and metal carbonyls.
For example a water molecule is a chemical bond of two hydrogen atoms and one oxygen atom. Solved examples for you. Ionic compounds and covalent compounds have different naming systems.
Water molecules are not absolutely neutral. Therefore the first step in naming a compound is to identify whether the compound is ionic or covalent. You would find ionic rather than covalent bonds in a salt such as sodium chloride.
When atoms connect with other atoms they are said to have a chemical bond. Magnesium chloride crystals sodium sulphate crystals solid leadii bromide diethyl ether hexane cyclohexane distilled water and naphthalene. Covalent compounds majorly have a very slow rate of reactions unlike the various ionic compounds.
Because the ability to attract electrons is so different between the atoms its like one atom donates its electron to the other atom in the chemical bond. Ionic bonds form between two atoms that have different electronegativity values. Here are some differences.
Naming ionic and covalent compounds. Covalent compounds chemical. So for example you would not expect to find covalent bonds in a metal or alloy such as silver steel or brass.
At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids. They are very different types of compounds with distinct attributes. Properties of ionic and covalent compounds experiment aim.
To compare the properties of ionic and covalent compounds. Ionic bonds are important because they allow the synthesis of specific organic compounds. This creates a spectrum of polarity with ionic polar at one extreme covalent nonpolar at another and polar covalent in the middle.
:max_bytes(150000):strip_icc()/covalent-bond-58de6d0b3df78c51627d1783.jpg)
Ionic Vs Covalent Bonds Understand The Difference
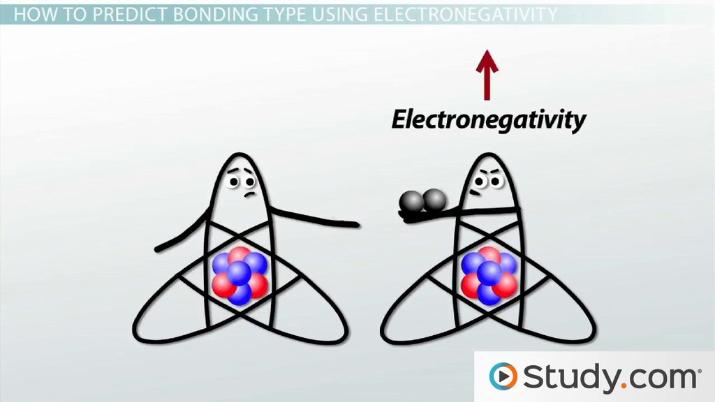
Polar And Nonpolar Covalent Bonds Definitions And Examples
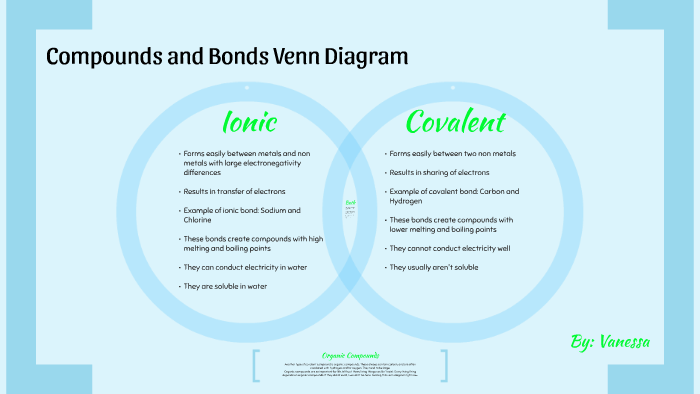
Ionic And Covalent Venn Diagram Bodum Westernscandinavia Org
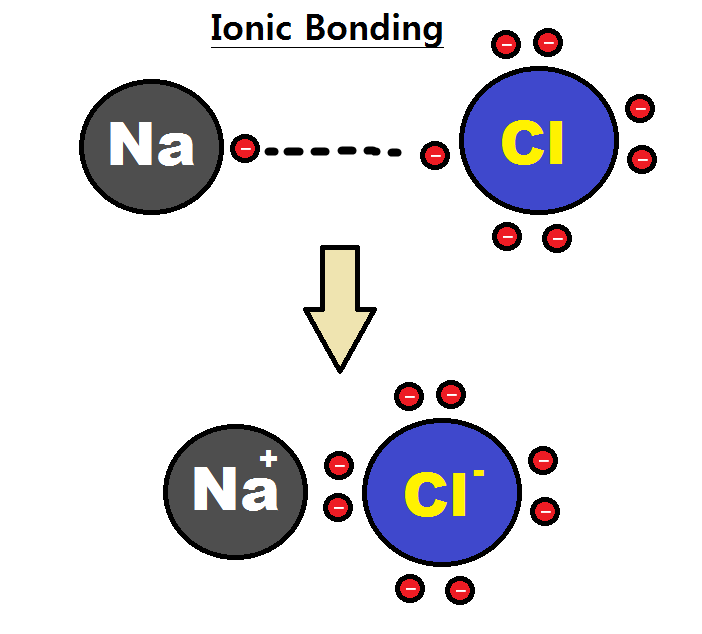
Difference Between Ionic And Covalent Compounds Compare The
/calcium-carbonate-molecule-536230216-57b066703df78cd39cdad367.jpg)
Compounds With Ionic And Covalent Bonds
Difference Between Ionic Covalent And Metallic Bonds Definition
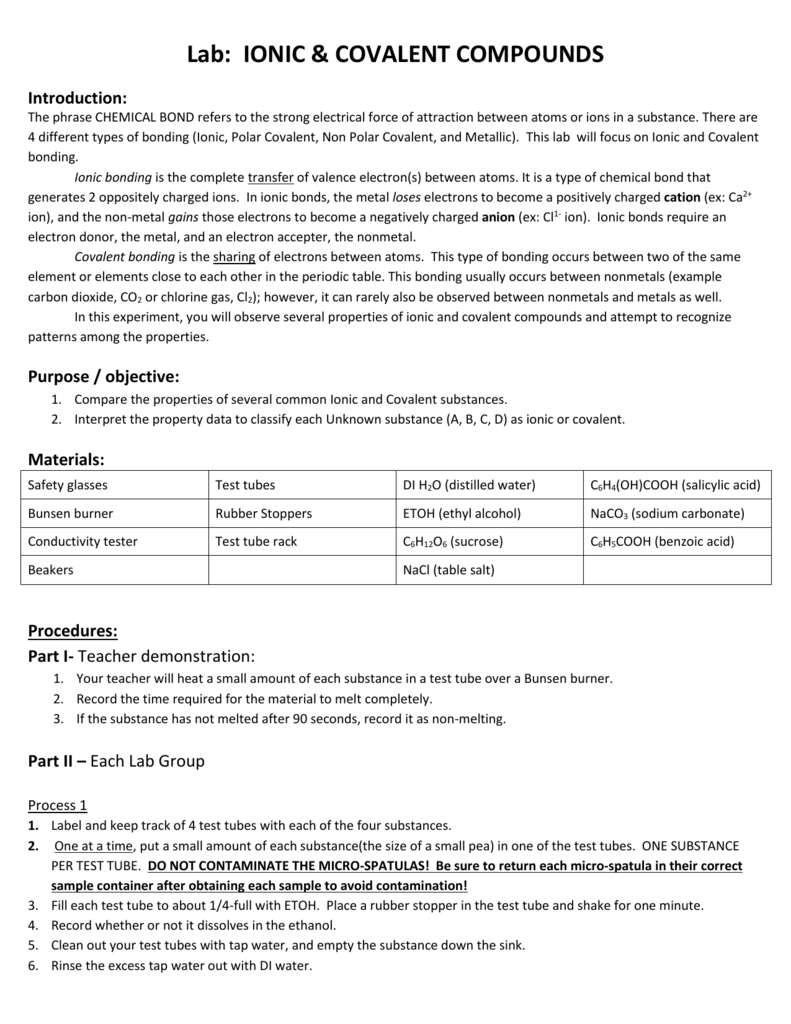
Ionic Covalent Lab
The Covalent Bond
Naming Compounds Names And Formulas Ppt Video Online Download
0 Response to "Ionic And Covalent Compounds Examples"
Post a Comment