Covalent Compounds Definition
Polarity is a measure of the separation of charge in a compound. These electron pairs are known as bonding electron pairs and they share these electrons to form covalent bond.
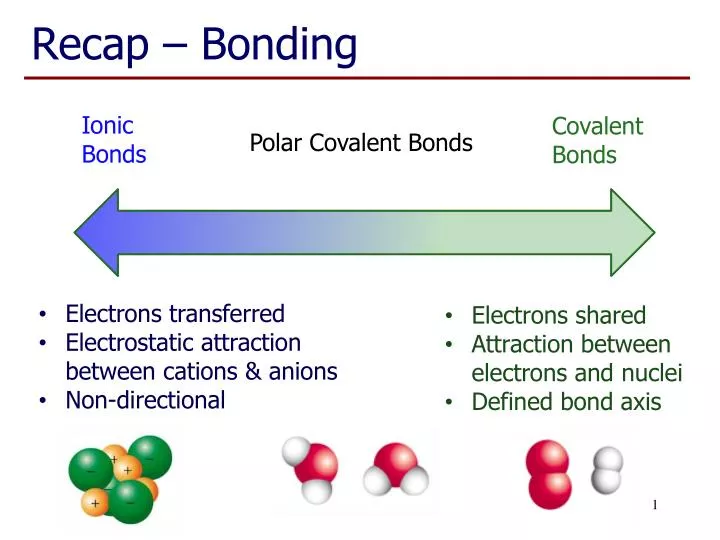
Ppt Recap Bonding Powerpoint Presentation Free Download Id
This type of bond may also be found in other chemical species such as radicals and macromolecules.

Covalent compounds definition. The elements form a compound by sharing electrons resulting in an electrically neutral molecule. A covalent bond may also be termed a molecular bond. Next time youre in the kitchen and have a moment to spare sprinkle some sugar on a dark surface and make some observations about this common tasty covalent.
For example most carbon based compounds are covalently bonded but can also be partially ionic. Polar covalent is the intermediate type of bonding between the two extremes. Covalent or molecular compounds generally result from two nonmetals reacting with each other.
However it can also be observed between nonmetals and metals. This bonding is primarily found between nonmetals. Covalent compound synonyms covalent compound pronunciation covalent compound translation english dictionary definition of covalent compound.
Covalent bond definition a covalent bond is a chemical bond that involves the sharing of electron pairs between two atoms. The elements form a compound by sharing electrons resulting in an electrically neutral molecule. Covalent definition the number of electron pairs that an atom can share with other atoms.
Definition of covalent compounds in the medical dictionary by the free dictionary. Properties of covalent compounds. Covalent bond definition the bond formed by the sharing of a pair of electrons by two atoms.
A chemical bond formed by the sharing of one or more. Covalent bond in a water molecule each hydrogen atom shares an electron with the oxygen atom. A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atoms.
Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
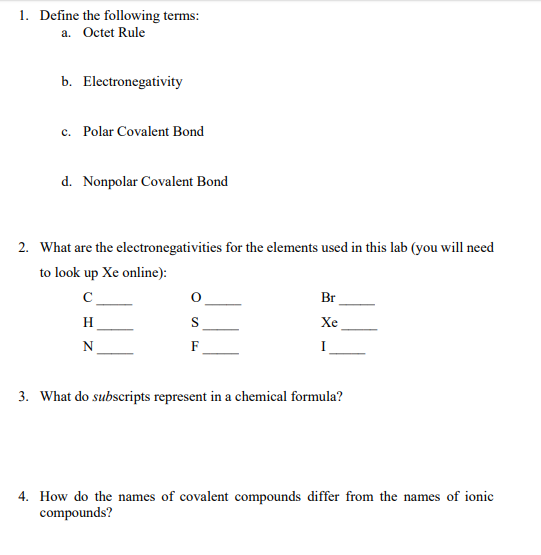
Solved 1 Define The Following Terms A Octet Rule B El
Single And Multiple Covalent Bonds Article Khan Academy

Chemical Bonds I Covalent Video Lesson Transcript Study Com
Covalent Bond Examples List
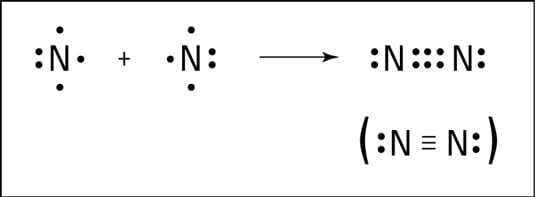
Multiple Bonds In Covalent Bonding Dummies
Difference Between Non Polar And Polar Covalent Bonds Difference

Bonding Review Define Ionic And Covalent Bonding Discuss Ionic

Lesson 37 Covalent Compounds Objectives The Student Will Define

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
0 Response to "Covalent Compounds Definition"
Post a Comment