Chemical Formula Of Rusting Of Iron
Iron metal is relatively unaffected by pure water or by dry oxygen. The collapse of the silver bridge in 1967 and the mianus river bridge in 1983 is attributed to the corrosion of the steeliron components of the bridge.
Physical Change Chemical Change Rusting Of Iron And Crystalization
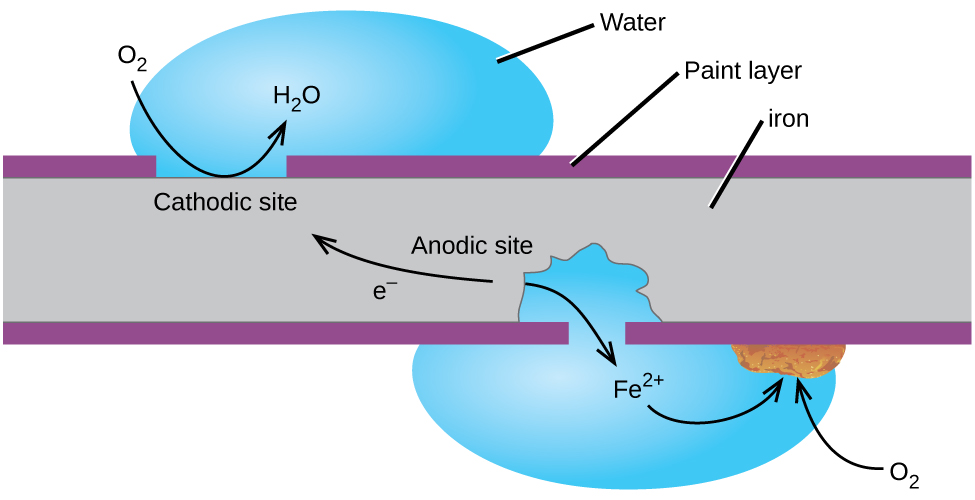
In this case electrons are being transferred from the iron to the oxygen.
Chemical formula of rusting of iron. Writing the balanced equation if youre interested in writing a balanced equation for the entire process you need only know the initial reactants and the products of the reaction. Rusting is the corrosion of iron and readily occurs in the alloy steel. Copper iron and aluminum metals all corrode over time loosing strength lustre and electrical conductivity.
When iron loses three of its electrons to oxygen it becomes the fe 3 ion and oxygen becomes the o 2 ion. Steel is an alloy made of iron and carbon. Rust is the common name for iron oxidethe most familiar form of rust is the reddish coating that forms flakes on iron and steel fe 2 o 3 but rust also comes in other colors including yellow brown orange and even greenthe different colors reflect various chemical compositions of rust.
The iron in the nail reacts with water and oxygen to produce rust a compound with the chemical formula fe3o2nh2o. The formation of a reddish brown flakes which loosely adheres to the iron is called rust. When impure iron is in contact with water oxygen other strong oxidants or acids it rusts.
The rusting of iron. Its an example of a chemical change. The fe 3 ions also react with water to form iron oxide fe 2 o 3 or rust.
This compound dehydrates to become fe 2 o 3h 2 o which is the chemical formula for rust. A nail rusting is a chemical change. If salt is present for example in seawater or salt spray the iron tends to rust more quickly as a result of electrochemical reactions.
The o 2 quickly reacts with h ions in water to form h 2 0. Rusting is a chemical process by which iron metal reacts with oxygen in the air to produce iron oxide otherwise known as rust. The rusting of iron can lead to damage to automobiles railings grills and many other iron structures.
Corrosion As An Electrochemical Process

The Formation Of Rust Reactions Of Metals With Oxygen Siyavula

Phases Commonly Found In Rust Layers Download Table
Spice Of Lyfe March 2019

12th Chemistry Project Rusting Of Iron

How Do You Remove Rust From Tools Science Abc

Rust Chemical Formula

17 6 Corrosion Chemistry
What Is The Formula Of Rust Quora

0 Response to "Chemical Formula Of Rusting Of Iron"
Post a Comment